1310-65-2
Lithium hydroxide
CAS: 1310-65-2
Molecular Formula: LiOH
1310-65-2 - Names and Identifiers
| Name | Lithium hydroxide |
| Synonyms | lithium hydrate Lithium hydroxide Lithiumhydroxideanhydrous LITHIUM HYDROXIDE 98+ 1 KG Lithium hydroxide anhydrous LITHIUM HYDROXIDE 98+ 100 G Lithium Hydroxide Anhydrous [for General Organic Chemistry] |
| CAS | 1310-65-2 |
| EINECS | 215-183-4 |
| InChI | InChI=1/Li.H2O/h;1H2/q+1 |
| InChIKey | WMFOQBRAJBCJND-UHFFFAOYSA-M |
1310-65-2 - Physico-chemical Properties
| Molecular Formula | LiOH |
| Molar Mass | 23.95 |
| Density | 1.43 |
| Melting Point | 470 °C (dec.) (lit.) |
| Boling Point | 925°C |
| Water Solubility | 113 g/L (20 ºC) |
| Solubility | water: soluble71g/L at 20°C |
| Appearance | Solid |
| Specific Gravity | 2.54 |
| Color | White to light yellow |
| Merck | 14,5534 |
| pKa | 14[at 20 ℃] |
| PH | 12 (50g/l, H2O, 50℃) |
| Storage Condition | Store below +30°C. |
| Stability | Stable. Incompatible with moisture. strong acids, carbon dioxide. |
| Sensitive | Air Sensitive & Hygroscopic |
| Use | For the preparation of lithium salt and lithium grease, alkaline battery electrolyte, lithium bromide refrigerator absorption liquid |
1310-65-2 - Risk and Safety
| Hazard Symbols | C - Corrosive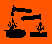 |
| Risk Codes | R52/53 - Harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment. R35 - Causes severe burns R20/22 - Harmful by inhalation and if swallowed. R22 - Harmful if swallowed R34 - Causes burns R23 - Toxic by inhalation |
| Safety Description | S45 - In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) S36/37/39 - Wear suitable protective clothing, gloves and eye/face protection. S26 - In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S22 - Do not breathe dust. |
| UN IDs | 2680 |
| WGK Germany | 1 |
| RTECS | OJ6307070 |
| TSCA | Yes |
| HS Code | 28252010 |
| Hazard Class | 8 |
| Packing Group | II |
| Toxicity | LD50 orally in Rabbit: 210 mg/kg |
1310-65-2 - Upstream Downstream Industry
| Raw Materials | Lithium carbonate |
| Downstream Products | Lithium bromide lithium metasilicate |
1310-65-2 - Reference Information
| NIST chemical information | information provided by: webbook.nist.gov (external link) |
| EPA chemical substance information | information provided by: ofmpeb.epa.gov (external link) |
| Introduction | lithium hydroxide is one of the most important lithium compounds, there are two kinds of anhydrous LiOH and LiOH · H2O. Anhydrous LiOH is a white tetragonal crystal particle or flowable powder with a relative density of 1.45g/cm3, a melting point of 471.2 ℃ and a boiling point of 1620 ℃. Lithium hydroxide monohydrate is a White deliquescent single crystal powder with a relative density of 1.51g/cm3 and a melting point of 680 ° C. When the temperature is higher than 100 ° C., the loss of crystal water becomes anhydrous LiOH. LiOH soluble in water, slightly soluble in alcohol, easy to absorb CO2 in the air to generate Li2CO3. LiOH and its concentrated solution are corrosive, and can corrode glass and ceramics at normal temperatures. |
| Application | LiOH is one of the main raw materials for the production of advanced lithium grease, lithium hydroxide is widely used, mainly used in chemical raw materials, chemical reagents, battery industry, petroleum, metallurgy, glass, ceramics and other industries, but also the defense industry, atomic energy industry and aerospace industry is an important raw material. Lithium grease produced by lithium hydroxide has long service life, strong water resistance, good fire resistance, difficult oxidation, and stable performance during multiple heating-cooling-heating cycles, the applicable temperature range can be from -50 ℃ to +300 ℃, which is widely used for lubrication of military equipment, aircraft, automobile, rolling mill and various mechanical transmission parts. In the battery industry, lithium hydroxide is used as an additive for alkaline storage batteries and nickel-hydrogen batteries, which can prolong the battery life and increase the power storage. |
| production method | lithium carbonate causticization method: the refined milk of lime is mixed with lithium carbonate. Mixing at a ratio of 08: 1, adjusting the concentration of the causticizing solution to 18g/ L to 20g/ L, heating to boiling and vigorous stirring, causticizing for about 30min. The reaction is as follows: the reaction can give a concentration of about 3. 5% LiOH solution. The insoluble residue was removed, separated, and the mother liquor was concentrated under reduced pressure and crystallized to obtain lithium hydroxide monohydrate. The lithium hydroxide monohydrate was dried at 130 ° C. To 140 ° C. And heated under reduced pressure at 150 ° C. To 180 ° C. To obtain anhydrous LiOH. The production of lithium hydroxide by causticization of lithium carbonate is currently the main method for the production of lithium hydroxide at home and abroad, especially abroad. |
| solubility in water (g/100ml) | grams dissolved per 100ml of water at different temperatures (℃): 12.7g/0 ℃;12.7g/10 ℃;12.8g/20 ℃;12.9g/30 ℃;13g/40 ℃;13.3g/50 ℃; 13.8g/60 ℃;15.3g/80 ℃;17.5g/100 ℃ |
| Application | is mainly used as a raw material for the preparation of lithium compounds. It can also be used in metallurgy, petroleum, glass, ceramic and other industries. for the preparation of lithium salt and lithium grease, alkaline battery electrolyte, lithium bromide refrigerator absorption liquid, etc. |
| category | corrosive article |
| toxicity grade | high toxicity |
| Acute toxicity | oral-mouse LD50: 200 mg/kg |
| storage and transportation characteristics | The warehouse is ventilated and dried at low temperature; It is stored separately from acids. |
| fire extinguishing agent | water, foam and sand mist |
| Occupational Standard | sel 1 mg/m3 |
Last Update:2024-04-10 22:29:15

Supplier List
Spot supply
Product Name: Lithium hydroxide Request for quotationCAS: 1310-65-2
Tel: +86-17551318830
Email: r@reformchem.com
Mobile: +86-17551318830
QQ: 3787852685
Wechat: chemical6666
Spot supply
Product Name: Lithium hydroxide Request for quotationCAS: 1310-65-2
Tel: +86-17551318830
Email: r@reformchem.com
Mobile: +86-17551318830
QQ: 3785839865
Wechat: chemical6666
Spot supply
Product Name: Lithium Hydroxide Visit Supplier Webpage Request for quotationCAS: 1310-65-2
Tel: +86-400-900-4166
Email: product@acmec-e.com
Mobile: +86-18621343501
QQ: 2881950922
Wechat: 18621343501
WhatsApp: +86-18621343501
Product Name: Lithium hydroxide Request for quotation
CAS: 1310-65-2
Tel: 0086-551-65418684
Email: sales@tnjchem.com
info@tnjchem.com
Mobile: 0086 189 4982 3763
QQ: 2881500840
Wechat: 189 4982 3763
WhatsApp: 0086 189 4982 3763
Product List: View Catalog
CAS: 1310-65-2
Tel: 0086-551-65418684
Email: sales@tnjchem.com
info@tnjchem.com
Mobile: 0086 189 4982 3763
QQ: 2881500840
Wechat: 189 4982 3763
WhatsApp: 0086 189 4982 3763
Product List: View Catalog
Spot supply
Product Name: Lithium hydroxide Request for quotationCAS: 1310-65-2
Tel: +86-17551318830
Email: r@reformchem.com
Mobile: +86-17551318830
QQ: 3787852685
Wechat: chemical6666
Spot supply
Product Name: Lithium hydroxide Request for quotationCAS: 1310-65-2
Tel: +86-17551318830
Email: r@reformchem.com
Mobile: +86-17551318830
QQ: 3785839865
Wechat: chemical6666
Spot supply
Product Name: Lithium Hydroxide Visit Supplier Webpage Request for quotationCAS: 1310-65-2
Tel: +86-400-900-4166
Email: product@acmec-e.com
Mobile: +86-18621343501
QQ: 2881950922
Wechat: 18621343501
WhatsApp: +86-18621343501
Product Name: Lithium hydroxide Request for quotation
CAS: 1310-65-2
Tel: 0086-551-65418684
Email: sales@tnjchem.com
info@tnjchem.com
Mobile: 0086 189 4982 3763
QQ: 2881500840
Wechat: 189 4982 3763
WhatsApp: 0086 189 4982 3763
Product List: View Catalog
CAS: 1310-65-2
Tel: 0086-551-65418684
Email: sales@tnjchem.com
info@tnjchem.com
Mobile: 0086 189 4982 3763
QQ: 2881500840
Wechat: 189 4982 3763
WhatsApp: 0086 189 4982 3763
Product List: View Catalog
View History
1310-65-2
链霉亲和素
154532-34-0
1022961-12-1
2-Methyl-5-(trifluoromethyl)benzonitrile
24165-03-5
1883-32-5
405-66-3
2807-30-9
3780-41-4
链霉亲和素
154532-34-0
1022961-12-1
2-Methyl-5-(trifluoromethyl)benzonitrile
24165-03-5
1883-32-5
405-66-3
2807-30-9
3780-41-4
Raw Materials for 1310-65-2
Downstream Products for 1310-65-2



